2022 Study: Adipose Therapy Significantly Improves Pain and Functional in Hip Osteoarthritis (OA)
Date: 17 February 2026
Admin: Medical Affairs
Living in the “Treatment Gap” With Hip Pain
For many active Canadians, hip pain is a silent thief. It often begins subtly — morning stiffness, a dull groin ache after a round of golf, or hesitation before a ski trip to Whistler. Over time, it starts to steal the things that define quality of life.
When you finally seek medical advice, the answer can feel disheartening. You may be diagnosed with hip osteoarthritis (OA) and told you are stuck in the “treatment gap.” You are not incapacitated enough to warrant an immediate total hip replacement, yet conservative treatments like physiotherapy, medications, or cortisone injections are no longer touching the pain. You are told to “wait until it gets worse.”
For people who view aging as something to be actively managed rather than endured, waiting is not a strategy. Fortunately, clinical research is beginning to address this gap.
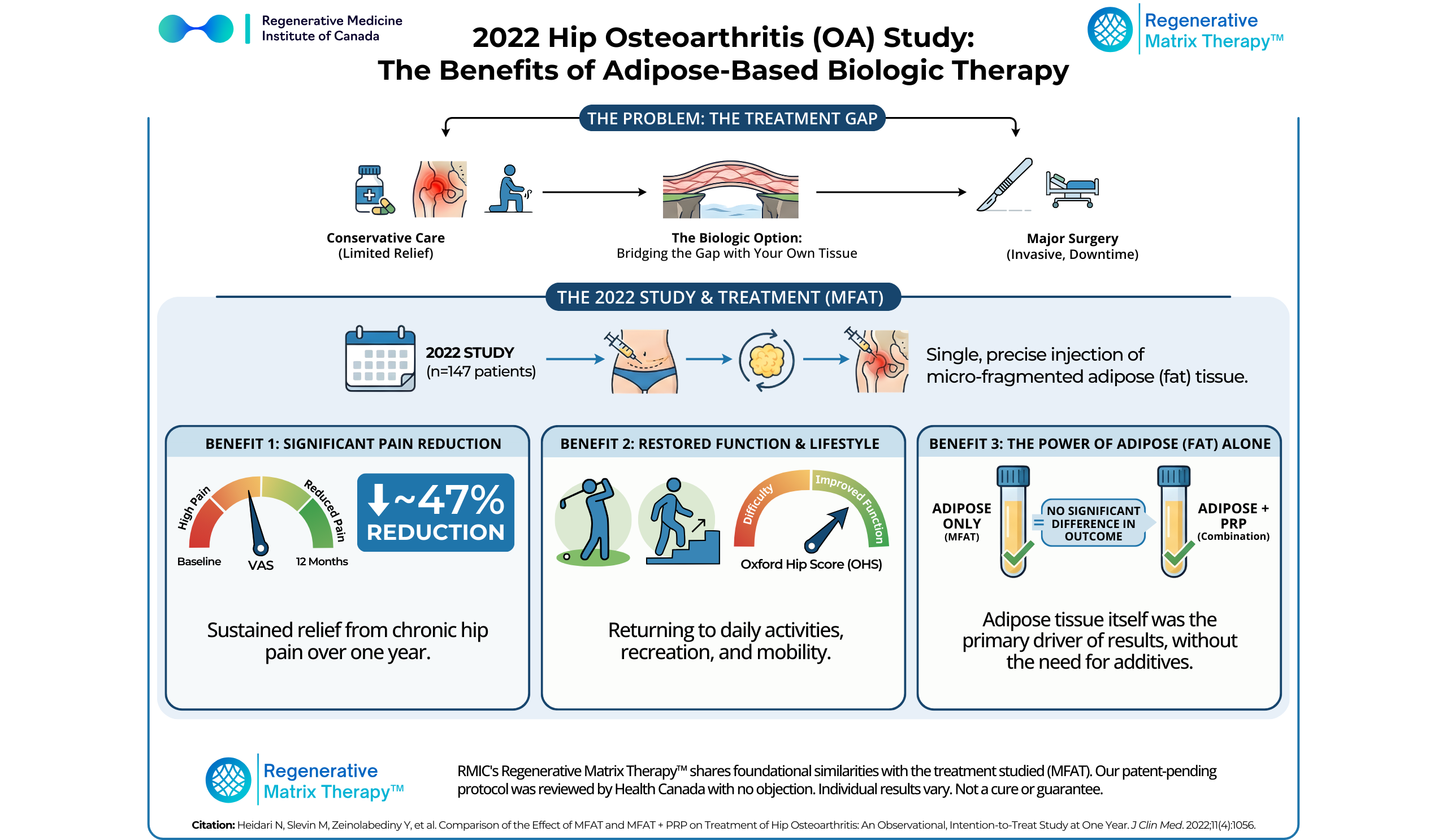
A pivotal 2022 clinical study published in the Journal of Clinical Medicine highlights a third option—a biologic pathway that uses the body’s own tissue to address hip pain without the scalpel. The researchers followed patients with hip osteoarthritis after a single ultrasound-guided injection of micro-fragmented adipose tissue (MFAT), with or without platelet-rich plasma (PRP), and tracked outcomes for a full year. In this study, ~42% of treated hips were KL grade 4, and ~64% were KL grade 3–4—a severity mix that’s particularly relevant for patients who feel like they’ve “run out of runway.”
Why Hip Osteoarthritis Is So Hard to Treat
Hip osteoarthritis presents a unique challenge compared to other joints.
The hip is a deep ball-and-socket joint surrounded by thick muscle layers. Inside the joint, articular cartilage allows for smooth movement, but cartilage has a critical limitation: it is avascular. It has no direct blood supply.
When cartilage deteriorates due to years of activity or gradual wear-and-tear, the body lacks an efficient pathway to deliver repair cells and growth factors to the damaged tissue. Without that supply line, inflammation compounds, stiffness increases, mobility declines, and pain becomes chronic.
Standard treatments do not solve this biological bottleneck:
- Pain medications mute symptoms without changing the joint environment
- Cortisone injections reduce inflammation but is toxic to cartilage over time
- Hyaluronic acid provides temporary lubrication without addressing the underlying biology
This is where adipose-based biologic therapies enter the conversation. By using a patient’s own fat tissue, clinicians can deliver a concentrated source of bioactive components directly into the joint, treating the underlying causes of disease.
Learn More About Regenerative Matrix Therapy™
Study Overview: MFAT vs. MFAT + PRP for Hip Osteoarthritis
In 2022, Heidari et al. conducted a large observational, intention-to-treat study evaluating MFAT injections for symptomatic hip osteoarthritis and MFAT combined with PRP.
Study Design
- Participants: 147 patients total (57 MFAT; 90 MFAT+PRP); average age ~65 with a wide age range (42–94) and a mix of active lifestyles.
- Severity Mix: ~42% of participants had KL Grade 4 arthritis (severe/bone-on-bone).
- Ranged from Kellgren-Lawrence (KL) grades 1 to 4 (mild to severe/bone-on-bone).
- Interventions:
- Group A: Received a single intra-articular injection of MFAT (Micro-fragmented Adipose Tissue).
- Group B: Received a combined injection of MFAT + PRP.
- Follow-Up: Outcomes were tracked for 12 months.
- Primary Metrics: Visual Analogue Scale (VAS) for pain and the Oxford Hip Score (OHS) for function.
Key Findings: Meaningful Improvement Without Surgery
The results were compelling because they provided evidence for the efficacy of adipose-based therapies in managing hip pain.
1. Significant and Sustained Pain Reduction
Both treatment groups experienced substantial pain relief that persisted through the one-year follow-up.
- MFAT group: ~37–47% pain reduction (mean VAS improvement: –20.8 points, decrease in the score indicates pain reduction)
- MFAT + PRP group: ~35% pain reduction (mean VAS improvement: –12.8 points)
Responder rates (pain, MCID ≥20-point VAS drop): Over 63% of patients were classified as “responders,” meaning they experienced clinically significant improvements.
- MFAT: ~63% (22/35 completers)
- MFAT + PRP: ~73% (32/44 completers)
2. Functional Improvement in Daily Life
The Oxford Hip Score (OHS), which measures the ability to perform daily tasks (like putting on socks, climbing stairs, or getting out of a car), showed marked improvement.
Function improved:
- MFAT: OHS improved by ~20% (30 → 36)
- MFAT + PRP: OHS improved by ~17% (29 → 34)
Overall, patients moved from scores indicating severe difficulty toward scores reflecting more manageable daily function; including tasks like climbing stairs, getting out of a car, or putting on socks.
3. The Power of Adipose Tissue (Fat) Alone
The numerical pain improvement was higher in the MFAT-only group, suggesting that the adipose tissue itself may be the primary driver of clinical benefit although the study found no statistically significant difference between MFAT alone and MFAT combined with PRP.
The adipose tissue itself is rich in mesenchymal stem cells (MSCs), pericytes, and a supportive collagen matrix. It appears that the adipose itself is the significant driver of the results. Adding PRP did not significantly enhance the outcome, validating the standalone power of adipose therapy.
4. Excellent Safety Profile
- No serious adverse events (infections or thromboembolic events) were reported across the entire trial
- The most common issues were transient hip pain and harvest-site discomfort; some patients required additional post-procedure pain medication.
What This Means for You
If you are living with hip osteoarthritis, these numbers translate into real-world changes:
- Less guarding with everyday movement
- More confidence walking, standing, and traveling
- Fewer trade-offs between activity and pain
Approximately 2 out of 3 MFAT patients and 3 out of 4 MFAT + PRP patients achieved a clinically meaningful reduction in pain by the study’s definition; and patients reported an average ~47% pain reduction.
This research reinforces the philosophy that your own body holds the key to its restoration. By injecting a small amount of your own fat tissue into the hip joint, we can help treat the underlying cause of disease – all without surgery.
- Avoid the Downtime: Unlike a hip replacement, which requires hospitalization and months of rehabilitation, this is a same-day procedure with no general anesthesia or hospital stay.
- Preserve Your Options: This therapy is designed to delay surgery, potentially for years, allowing you to maintain your native joint for as long as possible.
This study examined micro-fragmented adipose tissue (MFAT), a treatment that shares foundational similarities with Regenerative Matrix Therapy™, RMIC’s next-generation adipose-based biologic treatment. Our protocol was reviewed by Health Canada with no objection and is delivered under a physician-led framework focused on safety and patient selection.
For patients stuck in the treatment gap, not ready for surgery but no longer helped by conservative care, adipose-based biologic therapy may represent a meaningful third option.
Start Your Virtual Consultation
Research Highlights (For Clinicians)
- Study Citation: Heidari N, Slevin M, Zeinolabediny Y, et al. Comparison of the Effect of MFAT and MFAT + PRP on Treatment of Hip Osteoarthritis: An Observational, Intention-to-Treat Study at One Year. J Clin Med. 2022;11(4):1056.
- Study Design: Observational, intention-to-treat; two sequential cohorts
- Population: n = 147 hips; age range 42–94; KL grades 1–4; ~42% KL IV
- Intervention: Ultrasound-guided intra-articular MFAT ± PRP
- Follow-up: Pre-treatment, ~3 months, ~6 months, 12 months
- Endpoints: VAS (pain), OHS (function)
- Effect Sizes (12 Months)
VAS: –20.8 (MFAT); –12.8 (MFAT + PRP)
OHS: +6.57 (MFAT); +5.0 (approx.) MFAT + PRP - Responder Signal
Pain MCID responders: ~63% MFAT; ~73% MFAT + PRP (completers) - Safety: No serious adverse events reported
- Limitations
Non-randomized design
Observational nature
Loss to follow-up (~14–18%)
Learn More About Regenerative Matrix Therapy™See More Conditions We Treat
Disclaimer
Regenerative Matrix Therapy™ shares foundational similarities with, but is not identical to, the treatment studied. Individual results vary based on disease severity, anatomy, and adherence to post-care protocols. Educational content only; not a substitute for professional medical advice.
Why Choose Regenerative Matrix Therapy™ With RMIC?
At RMIC, Regenerative Matrix Therapy™ is delivered via a standardized, physician-led protocol built on years of orthobiologic experience and direct dialogue with Health Canada. Our adipose-based approach was reviewed by Health Canada with no objection and is built around safety, consistency, and realistic expectations.
If you are exploring advanced, non-surgical options to help manage hip osteoarthritis and preserve your lifestyle, find out whether Regenerative Matrix Therapy™ may be appropriate for you.