Evidence Summary: Adipose Therapy Provides Durable Relief for Partial Rotator Cuff Tears Without Surgery
Date: 18 November 2025
Admin: Medical Affairs
Introduction
Partial thickness rotator cuff tears are a common reason people give up tennis, pickleball, swimming, or even comfortable sleep. If you’ve been told you have a “partial tear” and advised to cycle between physiotherapy and cortisone shots while you “wait and see,” it can feel like your options are limited.
At RMIC, we follow the science closely. We’ve reviewed clinical trials of autologous adipose (fat)–based therapies for partial thickness rotator cuff tears, focusing on the outcomes that matter most: pain, shoulder function, quality of life, and the likelihood of avoiding surgery. Across published studies, a single adipose-based injection led to:
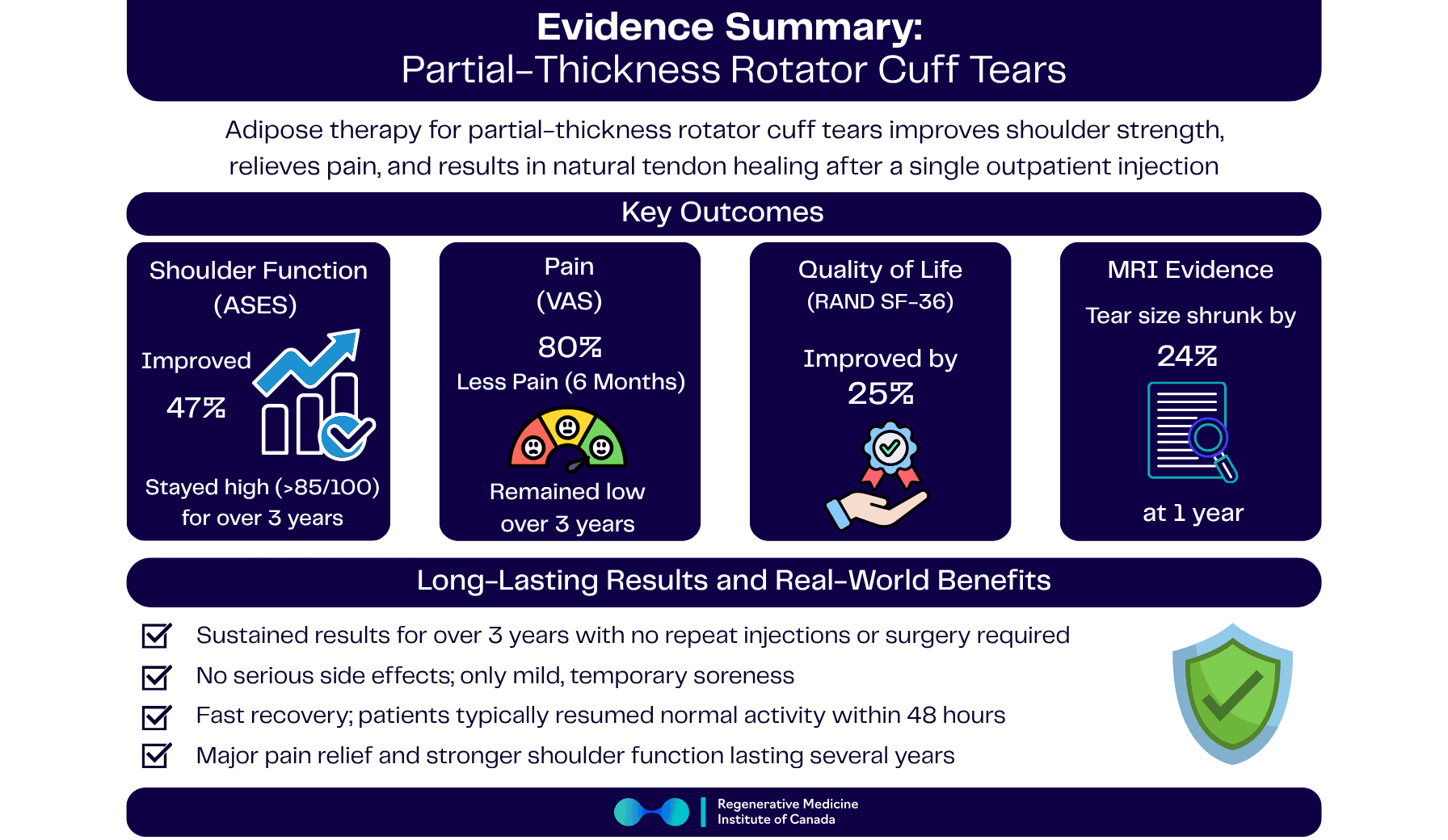
- Shoulder function improving by ~50% (ASES scores climbing from “limited” into the “excellent” range)
- Pain dropping by up to ~80% (VAS 2.6 → 0.5)
- Quality of life rising by ~25%
- Tear volume on MRI shrinking by ~23–24% on average
- Benefits that lasted more than three years, with no serious treatment-related adverse events and no progression to full-thickness tears in the adipose group
These trials examined autologous adipose-derived stem cell injections, a treatment that shares foundational similarities with Regenerative Matrix Therapy™, RMIC’s next-generation biologic treatment. Our treatment protocol was reviewed by Health Canada with no objection and is delivered in a physician-led setting focused on safety and outcomes. While not identical to Regenerative Matrix Therapy™, the overall signal is directionally supportive of our treatment philosophy.
Context / Problem
Partial thickness rotator cuff tears often present as:
- Pain with reaching overhead or behind your back
- Night pain when lying on the affected shoulder
- Weakness with lifting, serving, or throwing
- A slow, frustrating loss of confidence in your shoulder
Standard non-surgical management typically includes:
- Activity modification and rest
- Physiotherapy and rotator cuff strengthening
- Oral anti-inflammatories
- Corticosteroid injections
While these approaches can help, the relief is often temporary—especially with repeated steroid injections, which may carry concerns about long-term tendon health. Many patients are told to “wait until it’s bad enough” for surgery, such as arthroscopic repair or debridement, even though they’re still working, caring for family, and wanting to stay active in their 50s, 60s, and beyond.
This is exactly the gap where biologic, autologous tissue–based options are being explored: treatments that use your own tissue to support healing, with the goal of reducing pain, improving function, and potentially stabilizing or shrinking the tear without surgery.
Evidence Overview
The evidence summarized here comes from prospective clinical trials that treated MRI-confirmed partial thickness rotator cuff tears with a single injection of autologous adipose-derived stem cells, processed at the point of care and injected under imaging guidance.
Key features across the studies include:
- Population: Adults with symptomatic, MRI-confirmed partial thickness tears (no full-thickness ruptures)
- Intervention: One adipose-derived cell–based injection into the affected rotator cuff region
- Comparator: Corticosteroid injection in a randomized controlled design
- Follow-up: Detailed clinical and imaging follow-up through 6, 12, 33, and ~40–41 months (~3½ years)
- Outcomes:
- Shoulder function (ASES, 0–100)
- Pain intensity (VAS, 0–10)
- Whole-person quality of life (RAND SF-36 total, 0–800)
- MRI tear volume (mm³) and structural changes
Across these data, adipose therapy consistently outperformed corticosteroid injection on pain, function, quality of life, and structural integrity—with no adipose-treated shoulders progressing to full-thickness tears and no serious treatment-related adverse events reported.
While Regenerative Matrix Therapy™ is not identical to the stem cell products studied, it is a next-generation, autologous adipose-based treatment that shares foundational similarities. We view these results as directionally supportive of the type of biologic strategy we use, while recognizing that individual outcomes vary.
Key Findings
Magnitude of Improvement
- Shoulder function (ASES, 0–100)
- First 6 months: ASES scores rose from 59 → 86, a ~47% improvement in just half a year—well beyond the typical 12-point MCID for meaningful change.
- 1 year: Scores nudged higher to 89, locking in a ~52% improvement versus baseline and putting most patients into the “excellent” functional range.
- ~3½ years (40 months): Mean ASES values remained in the mid-80s, still ~50% above baseline, confirming that the early gains did not wash out over time.
- Pain intensity (VAS, 0–10)
- First 6 months: Pain dropped from 2.6 → 0.5, an ≈81% reduction, moving most shoulders into a “near-zero pain” zone.
- 1 year: A slight rise to 0.9 still represents a ~65% drop vs. baseline, keeping pain firmly in the mild range.
- Long-term: VAS scores remained low through the 33- and 40-month visits, sustaining ≥60% pain relief years after a single injection.
- Quality of Life (RAND SF-36 total, 0–800)
- First 6 months: SF-36 totals increased from 557 → 696, a ~25% improvement, reflecting broader gains in daily function, vitality, and comfort.
- 1 year: Scores held at 691, maintaining a ~24% advantage vs. baseline with no meaningful fade.
- ≈3 years: Mean values stayed well above 600, signalling that quality-of-life benefits tracked alongside sustained pain and function improvements.
- Tear volume on MRI (mm³)
- First 6 months: Average tear volume shrank from 58.6 → 45.0 mm³, a ~23% reduction in structural defect size.
- 1 year: Volume held at 44.5 mm³, consolidating a ~24% net shrinkage versus baseline and hinting at lasting biologic repair.
- Responder signal: About 55% of adipose-treated patients had their tear volume decrease by ≥10% at 12 months.
Durability of Results
- Multi-year stability: Functional gains and low pain scores were maintained through ~3½ years (mean 41 months) after a single procedure.
- No re-interventions in adipose group:
- Zero adipose-treated shoulders required surgery or repeat injections over multi-year follow-up.
- At 1 year, ASES scores remained ~89 with pain <1/10 and MRI tear volume still ~25% smaller than baseline.
- Long-term function:
- At 33 months, median ASES stayed in the mid-80s, well above clinically “normal” thresholds.
- At 41 months, adipose-treated shoulders still showed significantly higher mean ASES than baseline, indicating durable, long-lasting benefit.
Comparative Outcomes (vs. Corticosteroid Injections)
When adipose therapy was compared directly with corticosteroid injection, the differences were striking:
- Function: Adipose therapy yielded roughly double the functional improvement at 6 and 12 months versus steroid.
- Pain: Pain in the adipose group fell into the mild to near-zero range, while steroid patients often remained in the moderate pain range.
- Responder thresholds:
- 73% of adipose patients reached an ASES score ≥90 (“near-normal function”) at long-term follow-up, versus 20% in the steroid group.
- 46% of adipose patients achieved a perfect ASES 100 at least once; no steroid patient did.
- Only 40% of corticosteroid patients achieved a 50% reduction in pain, compared with 100% of adipose patients in the cited trial.
- Quality of life: Adipose therapy produced nearly double the quality-of-life improvement at 6 months and remained superior at 12 months.
- Structural integrity:
- Tear size shrank by ~23–24% in the adipose group.
- Tear size enlarged by ~22% in the corticosteroid group.
- Long-term advantage: At the 3-year checkpoint, ASES scores in adipose-treated shoulders were ~35% higher than those in the steroid group.
- Freedom from re-intervention: No adipose shoulder needed a repeat injection or surgery, whereas steroid-treated shoulders included cases requiring additional steroid or progression to full-thickness tear.
Responder Rates
- Near-normal function: 73% of adipose-treated patients achieved an ASES ≥ 90, typically associated with a symptom-free or nearly symptom-free shoulder.
- Perfect scores: 46% achieved a perfect ASES 100 at least once during follow-up.
- Pain responders:
- 100% of adipose patients reported meaningful pain relief (≥10-point drop) by 6 months.
- 100% reported pain ≤2/10 at follow-up in the trial.
- Structural responders: 55% had a ≥10% reduction in tear volume on MRI at 12 months.
Recovery & Treatment Experience
- Single outpatient procedure: Patients received a one-and-done adipose injection—no series of injections required.
- Point-of-care processing: Tissue was collected and processed during a single visit, then injected under image guidance.
- No general anesthesia: Procedures were done with local measures; a sling was used only for comfort.
- Quick return to life:
- Most patients returned to everyday activities within ~48 hours, with only light activity restrictions initially.
- No routine hospital stay or narcotic use was reported.
Safety & Tolerability
- No serious treatment-related adverse events were reported through more than 3 years of follow-up.
- Soreness but no major issues: Typical post-procedure soreness at the fat tissue harvest site and injection sites resolved quickly.
- Tendon safety:
- Zero adipose-treated shoulders progressed to full-thickness tears.
- In contrast, the steroid group included at least one clear progression to a full-thickness tear.
Overall, the safety profile in these early clinical trials appears favourable, particularly when weighed against the known limitations and potential downsides of repeated corticosteroid injections.
What This Means for You
If a partial thickness rotator cuff tear is stopping you from sleeping comfortably, playing your sport, or lifting without pain, these studies offer an encouraging picture of what an adipose-based biologic approach can achieve:
- Function: On average, shoulders worked ~50% better (ASES 59 → high 80s), with most patients landing in the “excellent” function range and three-quarters reaching near-normal scores.
- Pain: Pain levels dropped by ~65–80%, often into the 0–1/10 range—enough for many people to sleep, drive, and reach overhead without constant awareness of their shoulder.
- Quality of life: Whole-person health scores improved by ~25%, reflecting better participation in work, hobbies, and daily life.
- Tear size: Average tear volume shrank by ~23–24%, and over half of patients saw at least a 10% reduction on MRI.
- Durability: These benefits were still present 3+ years later, with no adipose-treated shoulders needing surgery or repeat injections in the trials reviewed.
At RMIC, Regenerative Matrix Therapy™ is our next-generation adipose-based treatment delivered under a standardized, physician-led protocol. The studies summarized above used autologous adipose-derived stem cell products that share foundational similarities with Regenerative Matrix Therapy™, but they are not identical to our protocol. Our approach was reviewed by Health Canada with no objection and is designed to combine careful patient selection, meticulous technique, and structured follow-up.
For many Canadians looking to avoid or delay rotator cuff surgery, especially those who want to stay active in golf, tennis, pickleball, or the gym, Regenerative Matrix Therapy™ may help bridge the gap between temporary measures and invasive procedures—while supporting better pain control, function, and long-term shoulder confidence.
Start Your Virtual Consultation
Research Highlights (Clinician Snapshot)
- Study Design: Prospective randomized controlled pilot trial and extended follow-up cohort using single autologous adipose-derived stem cell injections for partial thickness rotator cuff tears; corticosteroid comparator arm; follow-up to ~40–41 months.
- Population / Severity: Adults with symptomatic, MRI-confirmed partial thickness tears (no full-thickness ruptures); baseline ASES ≈ 59 (moderate impairment).
- Effect Sizes:
- Function (ASES 0–100): 59 → 86 at 6 mo (~47%↑), 59 → 89 at 12 mo (~52%↑); mid-80s at ~40 mo (sustained ~50%↑).
- Pain (VAS 0–10): 2.6 → 0.5 at 6 mo (~81%↓), 2.6 → 0.9 at 12 mo (~65%↓), low and stable at 33–40 mo.
- Quality of Life (SF-36 total 0–800): 557 → 696 at 6 mo (~25%↑), 691 at 12 mo (~24%↑).
- Tear Volume (mm³): 58.6 → 45.0 at 6 mo (~23%↓), 44.5 at 12 mo (~24%↓); 55% with ≥10% reduction.
- Responder Signal: 73% with ASES ≥ 90; 46% with ASES 100; 100% with ≥10-point VAS improvement and VAS ≤ 2/10 at follow-up.
- Comparator Performance: Adipose outperformed corticosteroid on ASES, VAS, SF-36, and MRI, with ~35% higher ASES at 3 years; steroid group showed tear enlargement (~22%↑) and included progression to full-thickness tear.
- Safety: No serious treatment-related AEs; no adipose-treated shoulders progressed to full-thickness tear; no re-interventions over 3+ years in adipose arm.
- Limitations: Small sample size, early-phase design, cell-derived product not identical to Regenerative Matrix Therapy™, MRI-based structural assessment, and restricted to partial thickness tears in adults.
Learn More About Regenerative Matrix Therapy™See More Conditions We Treat
Citations
- Hurd JL, Facile TR, Weiss J, et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study. J Orthop Surg Res. 2020;15(1):122. doi:10.1186/s13018-020-01631-8
- Lundeen M, Hurd JL, Hayes M, et al. Management of partial-thickness rotator cuff tears with autologous adipose-derived regenerative cells is safe and more effective than injection of corticosteroid. Sci Rep. 2023;13(1):19348. doi:10.1038/s41598-023-46653-4
Disclaimer
Regenerative Matrix Therapy™ shares foundational similarities with, but is not identical to, the adipose-based treatments summarized in this evidence review. Individual results vary. Educational content only; not a substitute for professional medical advice.
Why Choose Regenerative Matrix Therapy™ with RMIC?
Regenerative Matrix Therapy™ at RMIC is delivered under a standardized, physician-led protocol that emphasizes safety, evidence, and long-term outcomes. Our adipose-based approach was reviewed by Health Canada with no objection, and every patient undergoes a thorough evaluation to determine candidacy. We focus on helping you stay active, independent, and high-functioning, using advanced biologic treatments that align with your goals and values.
If you’re exploring non-surgical options for a partial thickness rotator cuff tear, we’re here to help you understand your choices and decide whether Regenerative Matrix Therapy™ fits into your care plan.