Wrist Osteoarthritis (OA) Evidence Summary: Adipose Therapy Shows Durable Relief
Date: 10 November 2025
Admin: Medical Affairs
Introduction
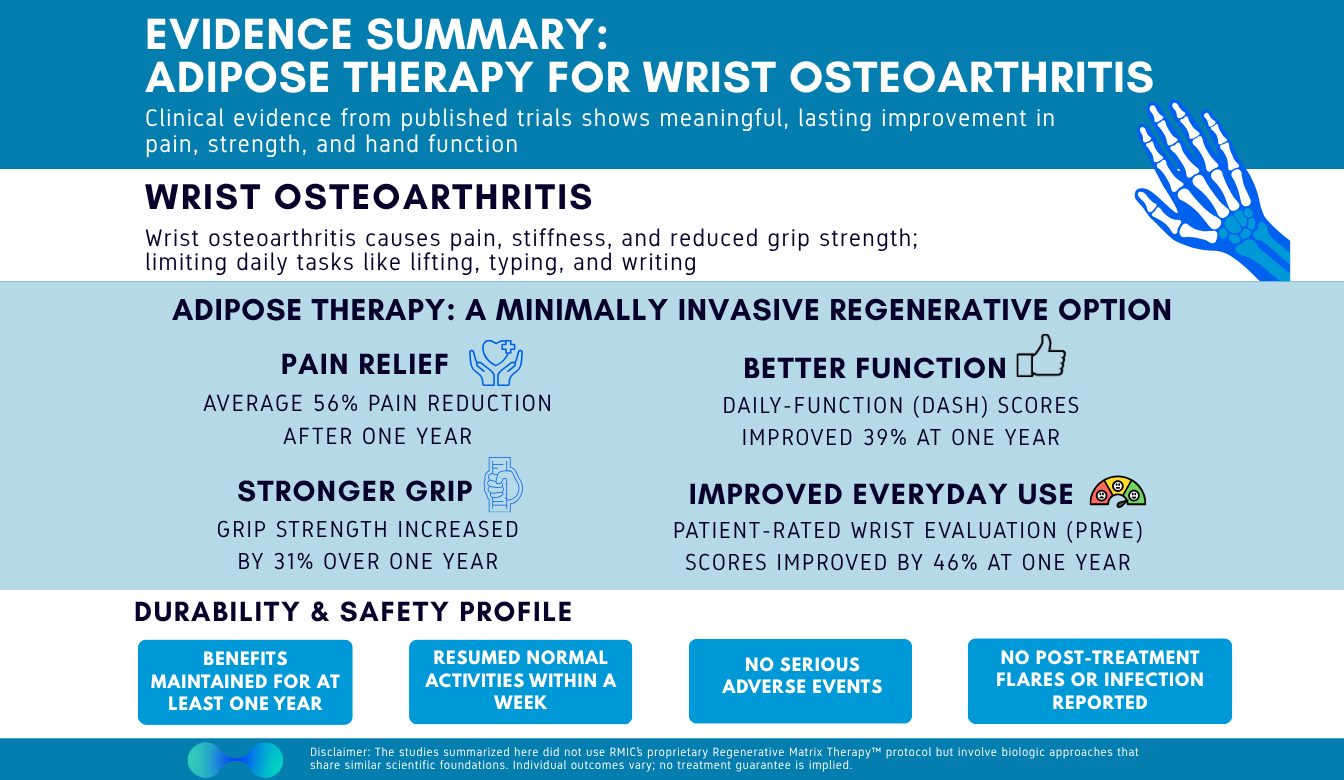
Wrist osteoarthritis can turn simple, daily tasks into uphill battles – but non-surgical options are evolving. At RMIC, we track the science closely. We’ve reviewed the peer-reviewed literature and synthesized an evidence summary of adipose therapy for wrist osteoarthritis, focusing on the outcomes patients care about most. Across published cohorts, patients saw significant pain reductions (over 50%), meaningful functional improvement (DASH/PRWE), and grip strength increase by over 30%; all with quick return to normal activity and no serious adverse events reported. The studied approaches use adipose-based techniques that share foundational similarities with Regenerative Matrix Therapy™, RMIC’s next-generation biologic treatment; while not identical, the overall signal is clearly supportive of our treatment philosophy. We break down the evidence – magnitude of improvement, durability, responder rates, recovery experience, and safety – so you can make a confident, informed decision about your care and even delay or avoid surgery.
Context / Problem
Wrist (radiocarpal) osteoarthritis limits strength, range of motion, and confidence in the hand. Standard non-surgical care – activity modification, braces, NSAIDs, steroid injections – often brings only short-term relief. Many people are told to “wait until it’s bad enough for surgery.” The gap between temporary measures and surgery is exactly where biologic, autologous tissue–based options are being explored.
Evidence Overview
Across multiple clinical cohorts of adults with symptomatic wrist osteoarthritis, a single adipose injection – with or without platelet-rich plasma – was associated with substantial and durable improvements in pain, function (DASH/PRWE), and grip strength, starting as early as 3 months and lasting at least 12 months. Imaging signals in many patients suggested potential structural benefit (cartilage or bone-marrow-edema changes). There were no serious adverse events across all trials and even post-procedure soreness was mild. While individual study protocols varied, the aggregate below provides a practical snapshot of what patients and clinicians can expect from this type of biologic approach.
Key Findings
Magnitude of Improvement
- Pain reduced by about half – and quickly. Average pain improvement approached ~50% within the first 3 months, ~53% at 6 months, and ~56% at 12 months after a single treatment (0–100 scale).
- Meaningful functional gains you can feel.
- Arm/hand function (DASH) improved by ~30-40%, with early improvement that continued to build over the year.
- Wrist pain/function (PRWE) also improved early and kept improving through 12 months – almost 50% improvement
- Grip gets stronger. Injured-side grip strength increased ~31% by 12 months (~8 kg gain), supporting better lifting, carrying, and daily confidence.
Durability of Results
- Quick relief that lasts. Pain, function, and strength improvements were seen 3 months after a single injection and continued to improve through 12 months.
- Severe OA still responds. All patients with the most advanced disease reached ≥ 50% pain reduction by 3 months, and about two-thirds kept that level of relief after 1 year.
- Structural signals (imaging). MRI findings showed cartilage or bone-marrow-edema improvements in a majority of patients over time.
Responder Rates
- DASH MCID achieved by ~75% of patients at 12 months.
- PRWE MCID achieved by ~92% of patients at 12 months.
- Pain responder threshold: ~58% of patients reached a ≥ 30-point reduction on a 0–100 pain scale at 12 months.
- Severe-OA milestones: 100% were ≥ 50% better by 3 months; ~67% maintained that milestone at 12 months.
Recovery & Treatment Experience
- Back to life fast. Most patients resumed normal activities within a week.
- Typical sensations: brief injection-site discomfort and harvest-site soreness (usually < 1 week) managed with simple, non-opioid pain medicine.
Safety & Tolerability
- No serious adverse events reported across the included patient groups.
- No post-injection flares requiring intervention; no neurovascular injuries, heterotopic ossification, or tumour signals seen through 12 months.
- Infection risk appeared low. Two lab batches showed culture contamination, but no clinical infections occurred after preventive antibiotics – a useful reminder of sterile-technique vigilance without evidence of downstream harm in these reports.
What This Means for You
If wrist osteoarthritis is shrinking your world – limiting strength, grip, or daily function – adipose therapy offers a minimally invasive, same-day procedure that, in multiple published reports, has reduced pain by over 50, improved hand function (DASH/PRWE) significantly, and increased grip strength by 31%. Most patients experience early improvements, including in severe OA, and many patients sustain benefits through a year.
At RMIC, Regenerative Matrix Therapy™ is our next-generation adipose-based treatment delivered under a standardized, physician-led protocol. The studies summarized above used adipose-based techniques that share foundational similarities with Regenerative Matrix Therapy™ – and some included platelet-rich plasma – yet they are not identical to our protocol. Our approach was reviewed by Health Canada with no objection, and it’s designed to pair careful patient selection with meticulous technique and follow-through. For many Canadians looking to delay or avoid wrist surgery, Regenerative Matrix Therapy™ may help bridge the gap between short-term measures and invasive procedures – while keeping you active in the routines you love.
Start Your Virtual Consultation
Research Highlights
Evidence Base: Multi-study synthesis of autologous adipose-based intra-articular injections for wrist (radiocarpal) OA; some cohorts combined adipose with PRP. Single-treatment protocols with follow-ups to 12 months; MRI used in select cohorts.
Representative Effects (Aggregated):
• Pain: ~53% reduction at 6 mo; ~56% at 12 mo (0–100 scale)
• Function: DASH ~30–35-point improvement (MCID ≈ 11); PRWE improved with 92% meeting MCID at 12 mo
• Strength: Grip +31% (~8 kg) at 12 mo
• Responders: ~58% reached ≥ 30-point pain reduction at 12 mo; in severe OA, 100% ≥ 50% pain reduction by 3 mo, ~67% maintained at 12 mo
Imaging: Majority showed cartilage or bone-marrow-edema improvements over time (cohorts with MRI).
Safety Snapshot: No serious AEs reported; no clinical infections observed (rare lab-culture contamination episodes managed with prophylactic antibiotics).
Learn More About Regenerative Matrix Therapy™See More Conditions We Treat
Citations:
- Mayoly A, Iniesta A, Curvale C, et al. International Journal of Molecular Sciences. 2019;20(5):1111. doi:10.3390/ijms20051111
- Mayoly A, Witters M, Jouve E, et al. Journal of Clinical Medicine. 2022;11(19):5786. doi:10.3390/jcm11195786
Disclaimer
Regenerative Matrix Therapy™ shares foundational similarities with, but is not identical to, the adipose-based treatments summarized in this evidence review. Individual results vary. Educational content only; not a substitute for professional medical advice.