2020 Trial: Adipose Injection Helped Even in Severe Knee Osteoarthritis (OA)
Date: 3 November 2025
Admin: Medical Affairs
Introduction
If you’ve been told you have severe knee osteoarthritis – often described on X-ray as Kellgren-Lawrence grade 3 or 4 (meaning advanced joint-space narrowing or “bone-on-bone” changes) – you’re not alone. Severe knee osteoarthritis can turn simple things like stairs, sleep, or a short walk into daily hurdles, and many people want an option between “more pills” and “eventual surgery.”
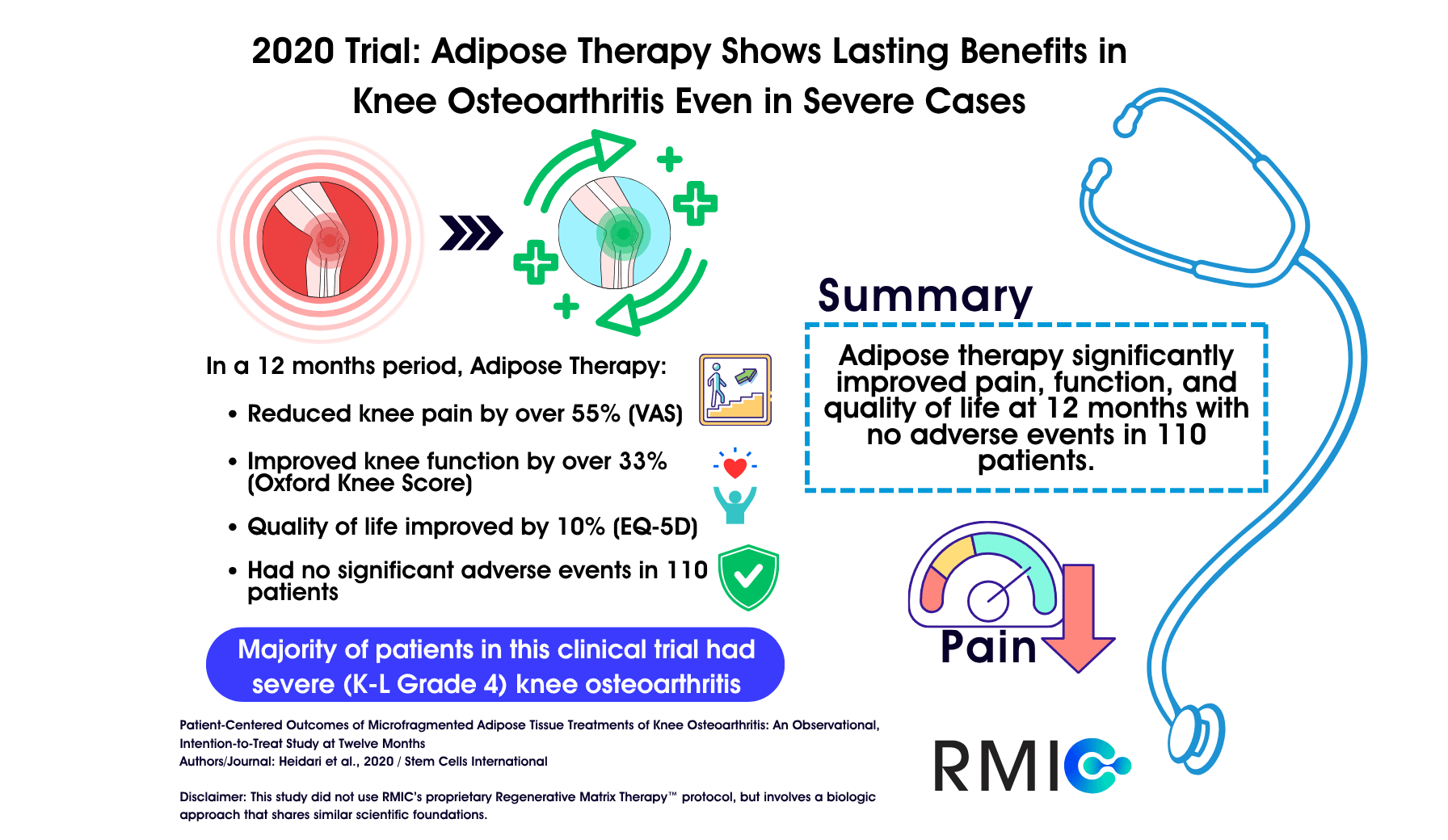
A 2020 clinical study followed patients – most with advanced disease – after a single, ultrasound-guided injection prepared from their own fat or adipose tissue and reported meaningful improvements in pain, function, and quality of life over 12 months. This study examined micro-fragmented adipose tissue (MFAT), a biologic treatment that shares foundational similarities with Regenerative Matrix Therapy™, RMIC’s next-generation biologic procedure. Our treatment protocol was reviewed by Health Canada with no objection and is delivered in a physician-led setting focused on safety and outcomes.
Context / Problem
Osteoarthritis is a whole-joint condition affecting cartilage, bone, synovium, tissue, ligaments, and surrounding muscles – and it’s a leading cause of disability as we age. Traditional choices often force a trade-off: short-lived relief versus invasive surgery. High-quality evidence in severe (Kellgren-Lawrence grade IV) OA has historically been relatively limited, which is why trials that enroll patients with advanced disease are particularly informative. In this study, 61.8% were grade IV and 80% were grade III–IV, underscoring that outcomes were observed in a population with predominantly severe disease.
Study Overview
- Design & Setting: Observational, intention-to-treat analysis at 12 months in a private practice; 110 knees received one ultrasound-guided intra-articular injection of autologous microfragmented adipose tissue (MFAT). Primary endpoints: pain (VAS), function (Oxford Knee Score, OKS), and quality of life (EQ-5D). Non-parametric paired Wilcoxon testing was used.
- Severity Mix: KL Grade IV (most severe) 61.8%; KL III 18.2%; overall 80% KL III–IV.
- Technique/Dose: A single intra-articular injection; ~8 mL MFAT delivered.
- Follow-up: Outcomes captured at baseline and 12 months; missing data were ~12% and treated as missing at random.
Key Findings
- Pain (VAS): Median pain improved by ~57% at 12 months (70 → 30; p < 0.001).
- Function (OKS): Median function score rose by ~34% (25 → 33.5; p < 0.001).
- Quality of Life (EQ-5D): Median health index increased by ~11% relative (+0.07 absolute; 0.62 → 0.69; p < 0.001).
- Responder Rate: 81% of patients experienced reduced pain and improved function after a single injection.
- Severe OA subgroup signal: In grade IV knees (most severe), medians improved for pain (VAS −32.5), function (OKS +7), and quality of life (EQ-5D +0.07) at 12 months – directionally positive results in the most advanced disease.
- Safety: No adverse events were reported (intra-operative, recovery, or postoperative).
What This Means for You
For people with knee osteoarthritis – including many with advanced (grade IV) changes – this real-world study found year-long improvements after a single adipose-derived injection. Translating the numbers into everyday terms:
- Pain dropped by roughly half (~57%, from 70 → 30), which for many patients means walking farther, sleeping better, and relying less on daily pain meds.
- Function improved by about one-third (~34%, OKS 25 → 33.5), often felt as easier stairs, longer outings, and greater confidence with day-to-day activities.
- Quality of life also improved (~11% relative, 0.62 → 0.69), consistent with feeling more active and less limited.
- Eight in ten (81%) patients reported both less pain and better function, an encouraging real-world “responder” signal.
Regenerative Matrix Therapy™ is RMIC’s next-generation, tissue-based biologic procedure designed to reduce pain and inflammation while supporting joint health. Regenerative Matrix Therapy™ is delivered through a standardized, physician-led protocol that Health Canada reviewed with no objection. This study examined microfragmented adipose tissue, a related approach that shares foundational similarities with RMIC’s treatment. While Regenerative Matrix Therapy™ is not identical to the intervention studied, the strong directionality of these results – including in severe OA – supports considering a carefully executed, next-generation adipose-based procedure to treat these conditions.
Start Your Virtual Consultation
Research Highlights
Study Design: Observational, intention-to-treat; n = 110 knees; single ultrasound-guided IA injection (~8 mL MFAT); outcomes at 12 months (VAS, OKS, EQ-5D); Wilcoxon paired testing.
Population Severity: KL IV 61.8%; KL III 18.2% (≈80% KL III–IV). Age 42–94.
Effect Sizes (Medians): VAS −40 (70 → 30; ~57%); OKS +8.5 (25 → 33.5; ~34%); EQ-5D +0.07 (0.62 → 0.69; ~11% relative); all p < 0.001.
Grade IV Subgroup: VAS −32.5; OKS +7; EQ-5D +0.07 at 12 months.
Responder Signal: 81% reported less pain + better function post-injection (author-reported composite).
Safety: No adverse events intra-op, recovery, or post-op.
Limitations: Non-randomized, no control group; PRO-based; missing data ~12% (treated as MAR); grades I–II small proportion, limiting formal subgroup inference.
Citation: Heidari N, Noorani A, Slevin M, et al. Patient-Centered Outcomes of Microfragmented Adipose Tissue Treatments of Knee Osteoarthritis: An Observational, Intention-to-Treat Study at Twelve Months. Stem Cells International. 2020;2020:8881405. doi:10.1155/2020/8881405.
Disclaimer
Regenerative Matrix Therapy™ shares foundational similarities with, but is not identical to, the treatment studied. Individual results vary. Educational content only; not a substitute for professional medical advice.
Notes for Clinically-Minded Readers
- The cohort used paired, non-parametric analysis appropriate for non-randomized, intention-to-treat data; ~12% missing at random were acknowledged.
- Improvements were seen across OA grades, including KL-IV, supporting biological plausibility for advanced disease, though control-free design limits causal inference.
- DOI for quick reference: 10.1155/2020/8881405.
Why choose Regenerative Matrix Therapy™ with RMIC?
Regenerative Matrix Therapy™ applies a standardized, physician-led protocol designed to harness the restorative potential of your own adipose tissue within a care pathway built for safety, consistency, and outcomes. While not identical to the intervention above, it shares core scientific principles and is performed in clinics operating under rigorous clinical governance. Our treatment protocol was reviewed by Health Canada with no objection, and candidacy is evaluated through a thorough consultation to align expectations, biomechanics, and rehabilitation for the best possible result.