2018 Study: Adipose Therapy Helped in Severe Knee Osteoarthritis (OA)
Date: 5 December 2025
Admin: Medical Affairs
Facing the “Bone-on-Bone” Crossroads
For many adults in their 50s, 60s, or beyond, an X-ray showing advanced knee osteoarthritis – often classified as Kellgren–Lawrence grade 3 or 4 – can feel like a loss of choice. The cartilage and tissues have eroded, the joint space has narrowed to almost nothing, and the next words often carry weight: “You’re bone-on-bone. It’s time for a knee replacement.”
Total knee arthroplasty can be highly effective, but it’s also major surgery with:
- Risks such as blood clots, infection, and nerve injury
- Recovery that can take months
- A real possibility (up to ~20%) of ongoing pain even after a technically successful replacement
- If you’re in your 50s or early 60s, you may worry about needing a revision later.
For those still active, whether that means hiking, skiing, or simply keeping up with grandkids, the idea of a full joint replacement can feel premature. Many start asking a reasonable question: Is there anything meaningful to try before surgery?
The Treatment Gap in Severe Knee OA
Knee osteoarthritis is one of the leading causes of pain and disability worldwide. In its early stages, there are more ways to manage it – physical therapy, activity modification, bracing, medications, platelet-rich plasma (PRP) or hyaluronic acid injections. But as the disease progresses to severe stages, these approaches lose effectiveness.
- Cortisone injections may provide temporary relief, but they can degrade the remaining cartilage and bone quality over time.
- Hyaluronic Acid (Gel) and Platelet-Rich Plasma (PRP) injections often become less effective as the joint space narrows.
- Surgery is effective for many, but it is invasive, irreversible, and carries significant risks.
That leaves thousands of patients in pain, taking opioids or NSAIDs just to get through the day, waiting until the pain becomes unbearable enough to justify surgery. This is the so-called “treatment gap”: when standard non-surgical care no longer helps, but surgery still feels like too big a step. Bridging that gap has become a major focus of orthobiologic research – leveraging the body’s own regenerative potential to support joint health.
A pivotal 2018 study published in American Journal of Orthopedics worked with patients who were already considered candidates for total knee replacement (“bone-on-bone” osteoarthritis) but instead received a single ultrasound-guided injection of their own micro-fragmented adipose (fat) tissue. Over one year, patients reported meaningful improvements in pain and function, with no serious adverse events. It suggests that even in cases of severe, “bone-on-bone” knee osteoarthritis, a precise injection of your body’s own adipose (fat) tissue can provide significant, durable relief.
This concept – using a patient’s own tissue to support joint function – shares foundational similarities with Regenerative Matrix Therapy™, RMIC’s next-generation biologic treatment. Reviewed with no objection by Health Canada and delivered in a physician-led setting, this approach represents a promising step toward safer, biologically grounded care for people facing advanced knee osteoarthritis.
For many, this emerging evidence offers something powerful: the hope that surgery may not always be the only path forward – and that improving pain and function could, quite literally, come from within.
Learn More About Regenerative Matrix Therapy™
Study Overview: Testing the Alternative to Surgery
Led by Dr. Jay Panchal and Dr. Gerard Malanga, this research focused specifically on the “tough cases” – patients who would typically be scheduled for a total knee replacement.
- Study Design: Prospective, single-center clinical study following patients for 12 months.
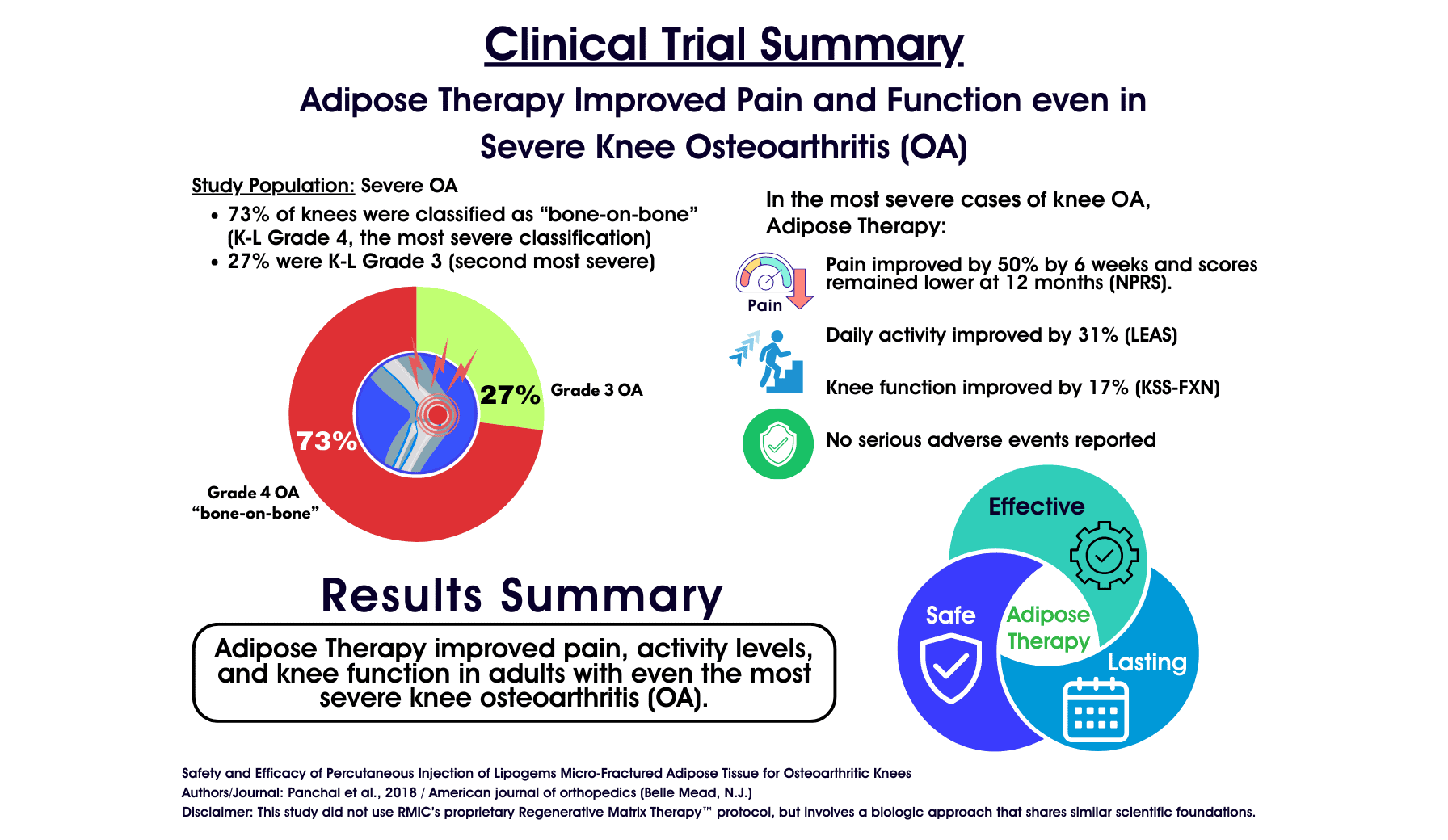
- Population: 17 subjects (26 knees) with Advanced Osteoarthritis.
- Severity Mix: The majority of knees (19 out of 26) were classified as Kellgren-Lawrence Grade 4—the most severe classification of “bone-on-bone” arthritis. The remaining 7 were Grade 3.
- The Problem: All patients had significant pain that was “refractory” (unresponsive) to conservative treatments like injections and physical therapy.
- Technique: Patients received a single, ultrasound-guided intra-articular injection of micro-fragmented adipose tissue (MFAT).
- Primary Goal: To assess if this single injection could provide safety and efficacy as an alternative to Total Knee Arthroplasty (TKA).
Key Findings: Meeting the Threshold of Relief
The results were compelling because they occurred in knees that were considered “too far gone” for other non-surgical treatments.
- Significant and Sustained Pain Reduction
Patients experienced a rapid drop in pain that was statistically significant and sustained for the full year of the study.- Rapid Relief: By 6 weeks, pain scores had dropped by approximately ~50%.
- Sustained Benefit: At the 1-year mark, pain scores remained significantly lower than baseline.
- The Stat: Pain improved by ~24% at 12 months (VAS 5.7 → 4.35; p = 0.017), with many patients experiencing much higher peak relief earlier in the year.
- People weren’t pain-free, but they were noticeably more comfortable.
- Functional Restoration (Exceeding the “MCID”)
In clinical research, we look for the Minimal Clinically Important Difference (MCID). This is the threshold where a patient actually feels a difference in their life – walking is easier, stairs are less painful, and daily tasks are manageable.- Function (FXN): Improved by ~17% (Score 65.4 → 76.4; p = 0.014).
- Knee Society Score (KSS): Improved by ~11% (Score 74.0 → 81.6; p = 0.014).
- The Verdict: The authors explicitly noted that the improvements in both function scores exceeded the MCID threshold. This means the change was a tangible improvement in quality of life for patients who were previously crippled by pain. People could walk, stand, and do daily tasks with less struggle.
- Improved Activity Levels
Beyond just pain and joint function, the study tracked the patients’ actual activity levels using the Lower Extremity Activity Scale (LEAS).- Getting Active Again: Activity scores showed significant improvement (~31%).
- Real-World Impact: This indicates that as pain diminished, patients were able to increase their physical engagement with the world – walking more, moving more, and returning to hobbies – during the critical months following treatment.
- Safety Profile
- No Serious Adverse Events: There were no infections, nerve injuries, or clots—complications commonly associated with knee replacement surgery.
- Minor Reactions: Some patients experienced minor pain or swelling from the injection that resolved within 48–72 hours.
What This Means for You: A Viable “Third Option”
If you have been told you have “severe”, “Grade 4”, or “bone-on-bone” knee OA, this study validates that you may still have options before committing to a metal knee.
In this study, a single targeted injection using a patient’s own fat:
- Pain eased by about one-quarter.
- Everyday function improved by around 15–20%.
- Actual activity levels (what people were doing out in the world) rose by around 30%.
In practical terms, that could mean:
- Walking a bit farther before you have to stop
- Getting through a round of golf or a vacation day with less pain
- Feeling more confident using your knee instead of constantly guarding it
Even for patients told they are “ready for surgery”, the authors concluded that a single adipose-based injection “may represent a nonsurgical treatment option to avoid knee joint replacement in this population”.
At RMIC, Regenerative Matrix Therapy™ is our next-generation adipose-based treatment. RMIC utilizes a proprietary Precision Adipose Modulation™ protocol, which shares foundational similarities with what was done in this study but it is not identical. Our protocol was reviewed by Health Canada with no objection and is delivered under a standardized, physician-led protocol, with careful screening to decide who is and isn’t a good candidate.
If you’re in that “in-between” zone – already being told to plan for a knee replacement but not ready to commit – it may be worth exploring whether Regenerative Matrix Therapy™ could be an option to help you stay active, independent, and doing the things you love.
Start Your Virtual Consultation
Research Highlights (for clinicians)
- Study Design: Prospective IRB-approved case series; 17 patients / 26 knees with refractory KL 3–4 OA; single ultrasound-guided intra-articular injection of autologous micro-fragmented adipose tissue (Lipogems®); outcomes at 6 weeks, 6 months, 12 months (NPRS, KSS, FXN, LEAS).
- Population Severity: All knees KL 3–4; 7 KL3, 19 KL4 (~73% KL4); mean age 68; mean BMI ~29 kg/m².
- Effect Sizes (12 months):
- NPRS: ~24% pain reduction (5.74 → 4.35)
- KSS: ~11% improvement (74 → 82)
- FXN: ~17% improvement (65 → 76)
- LEAS: ~31% improvement (36 → 47)
- Changes in KSS and FXN exceeded previously published MCIDs for TKA cohorts.
- Clinical Significance: Improvements in function scores exceeded the Minimal Clinically Important Difference (MCID), indicating tangible patient benefits
- Safety: No serious adverse events; transient pain and swelling resolving within 48–72 hours.
- Limitations: Small n, single-center; uncontrolled (no comparator); unclear handling of missing data; PRO-based endpoints only; potential placebo and expectation effects not excluded.
- Statistical approach: Paired t-tests comparing baseline vs 6 weeks, 6 months, and 12 months for NPRS, KSS, FXN, LEAS; significance defined at p < 0.05 (reported as “significant” without exact p-values)
- Citation: Panchal J, Malanga G, Sheinkop M. Safety and Efficacy of Percutaneous Injection of Lipogems Micro-Fractured Adipose Tissue for Osteoarthritic Knees. Am J Orthop (Belle Mead NJ). 2018;47(11). doi:10.12788/ajo.2018.0098.
Learn More About Regenerative Matrix Therapy™See More Conditions We Treat
Disclaimer
Regenerative Matrix Therapy™ shares foundational similarities with, but is not identical to, the treatment studied. Individual results vary. Educational content only; not a substitute for professional medical advice.
Why choose Regenerative Matrix Therapy™ with RMIC?
At RMIC, Regenerative Matrix Therapy™ is delivered via a standardized, physician-led protocol built on years of orthobiologic experience and direct dialogue with Health Canada. Our patent-pending, adipose-based approach was reviewed by Health Canada with no objection (not an approval), and is built around safety, efficacy, and realistic expectations.
If you’re exploring advanced, non-surgical options to help manage knee osteoarthritis and preserve your lifestyle, find out whether Regenerative Matrix Therapy™ is a good fit for you.
Start Your Virtual Consultation